Stability and Material Compatibility of Biopharmaceuticals
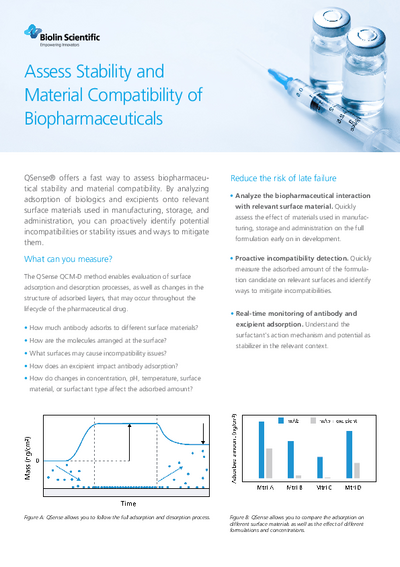
from QSenseQSense has developed a method to evaluate the effects of surfaces and interfaces on molecules ranging from small to complex. It helps to understand the risks of alteration of the concentration or efficacy of drugs and active molecules at an early stage of development and formulation processes. In the white paper “Using QSense QCM-D to assess Stability and Material Compatibility of Biopharmaceuticals” you can find more details.
Downloads
Navigation
Categories
Contact
Quantum Design
Krivoklatska 37
199 00 Praha 9
Czech Republic
| Phone: | +420 607 014 278 |
| E-mail: | czechiaqd-europe.com |