Solar-to-Hydrogen Efficiency (STH)
The development of a sustainable energy economy that is not based on limited fossil fuels but on renewable energies is an urgent task. Hydrogen is one of the leading alternatives for the storage and transportation of energy, provided that it can be stored safely and be produced efficiently using sustainable energy sources. The problems associated with electrical energy storage can be avoided as the energy is stored in the form of chemical bonds. Solar energy, the largest sustainable energy source, has the potential to provide enough power on the scale required for carbon dioxide-free hydrogen production. Direct solar water splitting using photocatalytic or photoelectrochemical processes is a promising alternative to the two-stage energy conversion of solar energy via electrical energy into chemical energy. This route has the long-term potential to become more economically attractive than the already established two-stage process. Today, there are neither materials nor reactors or cells that fulfill all the necessary requirements for an economical implementation of solar water splitting, as the complexity of photocatalytic or photoelectrochemical water splitting requires complicated component design and/or the use of rare and expensive materials.
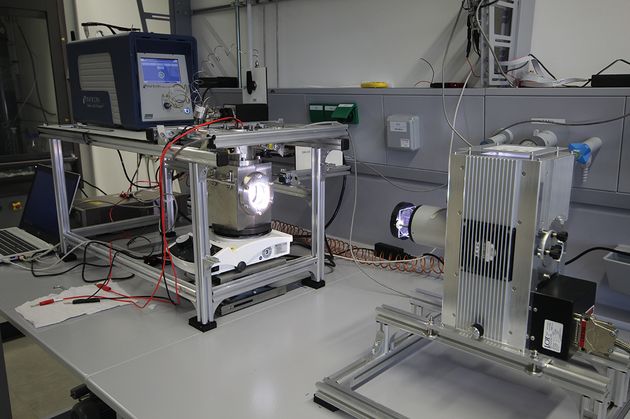
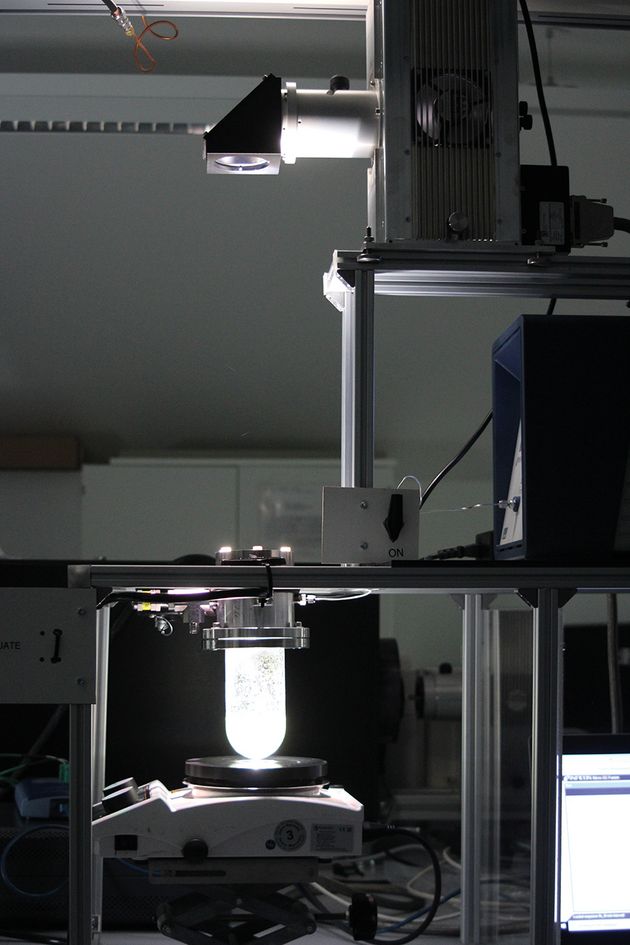
An essential step in the development of photocatalytically or photoelectrochemically efficient water splitting systems is, similar to solar cells, the implementation of standardized, comparable performance measurements. There is one parameter in particular that has established itself internationally: Solar-to-Hydrogen Efficiency (STH), i.e. the quotient of the chemical energy that can be released by burning the hydrogen produced and the irradiated solar energy. If the STH is not available, the photocurrent density at a voltage of 1.23 V is used as a performance criterion, particularly in photoelectrochemical water splitting. Irradiation of the global standard spectrum AM1.5G is necessary for both. In the Functional Materials working group, the combination of a xenon lamp and AM1.5G filter was used to create the necessary radiation conditions to determine the STH or the photocurrent density with the aid of a gas chromatograph or a potentiostat. As the energy density of the light is also important in addition to the spectral distribution, this must be set by using a calibrated solar cell. This can also be easily accomplished with the set-up described. This means that nothing stands in the way of reproducible characterization of photoelectrodes and photocatalysts in accordance with international standards.
Source: User report on the solar simulator from Quantum Design Europe University of Salzburg, Department of Chemistry and Physics of Materials, Functional Materials working group.
Contact
| +49 6157 80710-34 | |
| +49 6157 80710934 | |
| Write e-mail |