Lanthanides – Magnetic measurements on gadolinium and dysprosium
From a magnetic point of view, lanthanides are highly exciting. Furthermore, some of these elements are critical to produce permanent magnets with special characteristics.
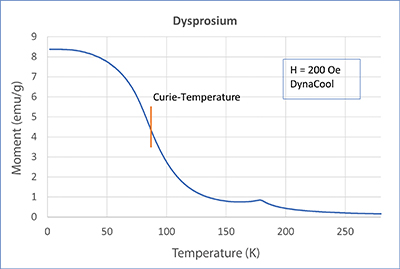
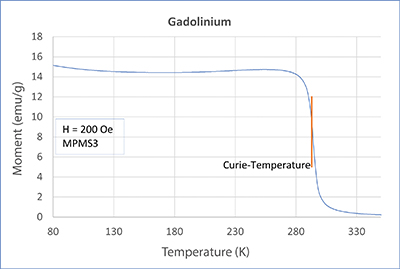
In this article, we will treat the two elements gadolinium (Gd) and dysprosium (Dy). In our application lab, we measured both metals with a SQUID magnetometer MPMS3 and PPMS-DynaCool with VSM option. Check out the measurement’s curves in the adjacent figures. Both elements show a strong transition. For Gd, this transition is at about 293 K, which is approximately room temperature. For Dy, on the other hand, the transition is at about 87 K. Both samples become ferromagnetic below the transition point. The corresponding temperature is called Curie temperature or Curie point, after the french physicist and Nobel Prize winner Pierre Curie.
Looking closely at the curves, you can see a little hump on the dysprosium measurements. This is another transition (paramagnetic - antiferromagnetic - AFM). It lies at approx. 180 K and is called Néel temperature, after the French physicist and Nobel prize winner Louis Néel. In an AFM state, spins organize themselves, so they eliminate each other. Therefore, when the temperature decreases, the magnetic moment also decreases (lower temperatures support the eliminating order). This is noticeable but overwritten by the developing ferromagnetism. From neutron scattering experiments we know that spins in dysprosium in the AFM state take on a spiral order and thus eliminate each other. The elements in the periodic system adjacent to dysprosium (Tb, Ho, Er, Tm) show a similar behavior (AFM, TNéel > TCurie). But what about gadolinium? Does it also show antiferromagnetism? Yes, it does! However, for Gd, the AFM state only exists at very small magnetic fields. There was no AFM state at the 200 Oe we used for the measurements.
If you found our little excursion interesting, please keep reading. We will continue with this topic in one of the next Spectrum issues. If you have comments or questions, please contact us!
Key facts:
Lanthanides or the lanthanoid series of elements are sometimes referred to as rare earth elements, which is not quite correct. Lanthanides are all 15 elements from lanthanum (atomic number 57) to lutetium (atomic number 71). Rare earth metals also contain the two transition elements scandium (Sc) and yttrium (Y). There is no known magnetic ordering of Sc and Y.
Contact
| +49 6157 80710-46 | |
| +49 6157 80710946 | |
| Write e-mail |