Stability and Material Compatibility of Biopharmaceuticals
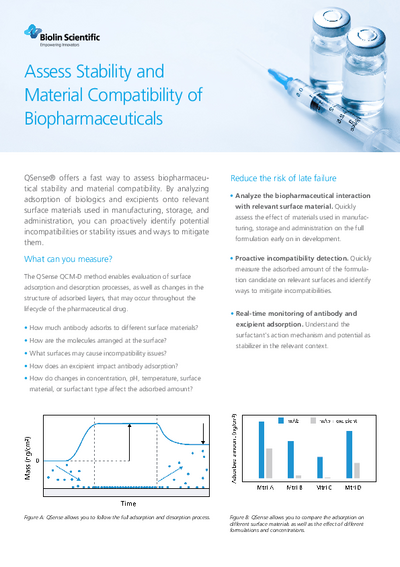
from QSenseQSense has developed a method to evaluate the effects of surfaces and interfaces on molecules ranging from small to complex. It helps to understand the risks of alteration of the concentration or efficacy of drugs and active molecules at an early stage of development and formulation processes. In the white paper “Using QSense QCM-D to assess Stability and Material Compatibility of Biopharmaceuticals” you can find more details.
Downloads
Contact

Dr.
Agnieszka
Kowalczyk-Głowacka
Compact and automated quartz microbalance
The quartz crystal microbalance Omni from QSense is the new state-of-the-art instrument from the pioneers of QCM-D. The mounting of the sensor is semi-automatic and allows the highest reproducibility ...
Flexible quartz crystal microbalance system
The Explorer from QSense is a compact design QCM-D for one flow module, holding one sensor. It is temperature controlled and samples are introduced by using a peristaltic pump included in the system. ...
Fully automated quartz crystal microbalance system
PRO is the high-end QCM-D surface analysis equipment. It is an intuitive instrument and software platform that senses mass change, layer thickness, binding and molecular orientation at the nano-scale. ...
Navigation
Categories
Contact
Quantum Design GmbH
ul. Sztygarska 12/3
41-500 Chorzow
Poland
| Phone: | +48 32 2482048 |
| Mobile: | +48 515 166893 |
| E-mail: | polandqd-europe.com |